Helium has the smallest atomic radius. This site reports that the atomic radius is 31xx10-12m where the atomic radius of the hydrogen atom is 53xx10-12m.
Atomic Radius Trend Periodic Table Chemtalk
From electron filling of atomic shells we know that the smallest atomic radius will be of the element which has electrons having lowest principal quantum n 1.

. The other elements in the p-block of the periodic table also have small atomic radii but helium is. This is due to trends in the periodic table and the effective nuclear charge that holds the valence electrons close to the nucleus. What elements have the smallest radius.
The atomic radius of a particular element is an important characteristic as it helps us to understand many properties. As can be seen in the figures below the atomic radius increases from top to bottom in a group and decreases from left to right across a period. Atomic size decreases across a Period but INCREASES down a Group.
Which has smallest radius. Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top. Agar boglanish kovalent bolsa atom yadrolari orasidagi masofani ikkiga boling.
That is all three ions contain 18 electrons but have different nuclear charges. That atomic size should decrease across a Period from left to right as we face the Table can. How do you calculate atomic radius.
Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top. Atoms further down the periodic table are larger because they have more shells of electrons Atoms farther to the right on the table are smaller because th. This is due to trends in the periodic table and the effective nuclear charge that holds the valence electrons close to the nucleus.
This is due to trends in the periodic table and the effective nuclear charge that holds the valence electrons close to the nucleus. Because K has the greatest nuclear charge Z 19 its radius is smallest and S 2 with Z 16 has the largest radius. Therefore the radius of atoms increases as you go down a certain group in the periodic table of elements.
Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top. An atom gets larger as the number of electronic shells increase. Atomic radii vary in a predictable way across the periodic table.
K Cl and S 2 form an isoelectronic series with the Ar closed-shell electron configuration. In general the size of an atom will decrease as you move from left to the right of a certain period. Helium has the smallest atomic radius at 31 picometers.
As such we have only two elements which fill the 1s orbital. Helium is in the top period and the farthest right group which follows the patterns of atomic radius on the periodic table. We know that Hydrogen atom has one proton in its nucleus whereas helium atom has two protons.
Answer 1 of 7. And thus the element with the smallest atomic radius should be helium Z2. Helium has the smallest atomic radius.
Which element has the smallest atomic radius. Misol uchun agar siz ikkita kovalent boglangan atom yadrolari orasidagi masofani 100 pikometr pm bilsangiz har bir alohida atomning radiusi 50 pm. Which ion has lowest atomic radius.
How do you find the smallest atomic radius. Helium has the smallest atomic radius. Thus helium is the smallest element and francium is the largest.
The element that is considered to have the smallest atomic radius is helium.
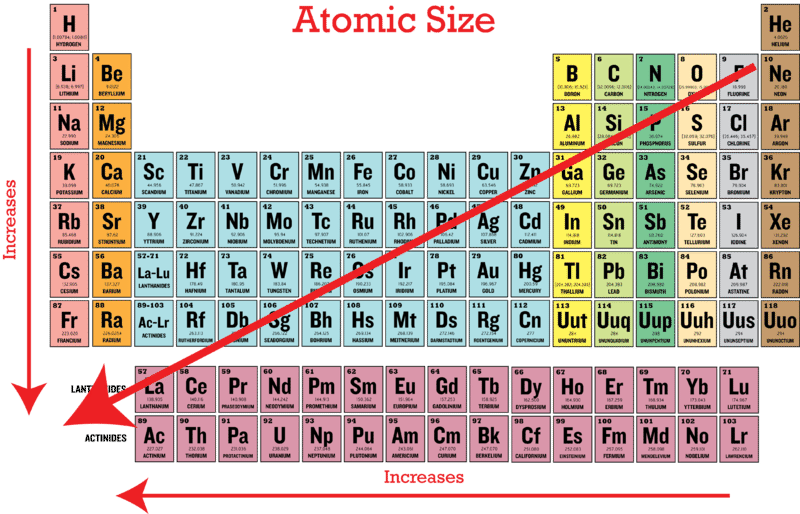
Periodic Trends In Atomic Size Ck 12 Foundation

Periodic Trends Determine Which Atom Has The Smallest Atomic Radii Radius Johnny Cantrell Youtube
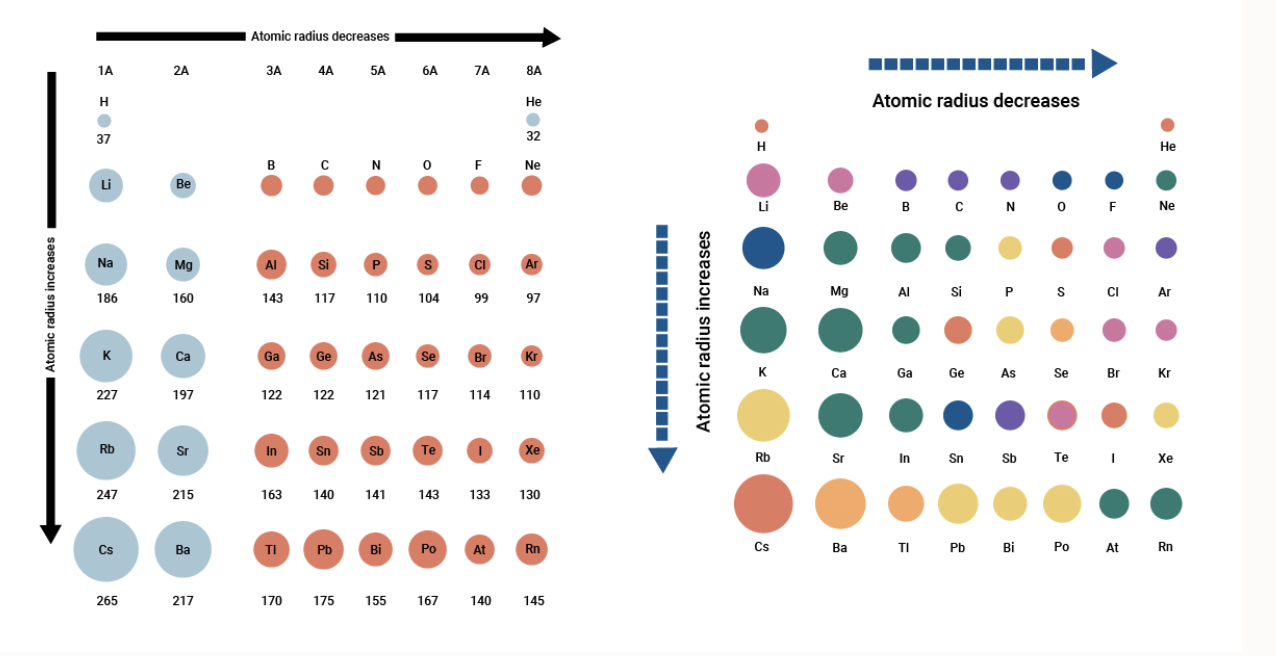
0 Comments